SI System of Units : The SI (International System) provides a single framework for all measurements. Engineers and scientists worldwide rely on it. By using fixed base units and clear prefixes, The SI system removes confusion. Consequently, everyone speaks the same “unit language” in calculations and designs.
Understanding Base Units
The SI system defines seven base quantities. Each has one unit:
- Length: meter (m)
- Mass: kilogram (kg)
- Time: second (s)
- Electric current: ampere (A)
- Temperature: kelvin (K)
- Amount of substance: mole (mol)
- Luminous intensity: candela (cd)
These base units form the building blocks for every other SI measurement.
Forming Derived Units
From base units, you derive all other units. For example:
- Force (newton, N): kg·m/s²
- Pressure (pascal, Pa): N/m² or kg/(m·s²)
- Energy (joule, J): N·m or kg·m²/s²
- Power (watt, W): J/s or kg·m²/s³
Using these formulas, you calculate complex values with simple unit algebra.
Using SI Prefixes
To handle very large or very small numbers, SI uses prefixes:
- kilo (k): 10³
- mega (M): 10⁶
- giga (G): 10⁹
- milli (m): 10⁻³
- micro (µ): 10⁻⁶
- nano (n): 10⁻⁹
For instance, 1 km equals 1 000 m, and 1 µA equals 0.000 001 A. This shorthand keeps numbers readable.
Advantages of SI System of Units
- It is a coherent system of units.
- It is a rational system of units.
- SI is a metric system.
SI System of Units : Converting Between Units
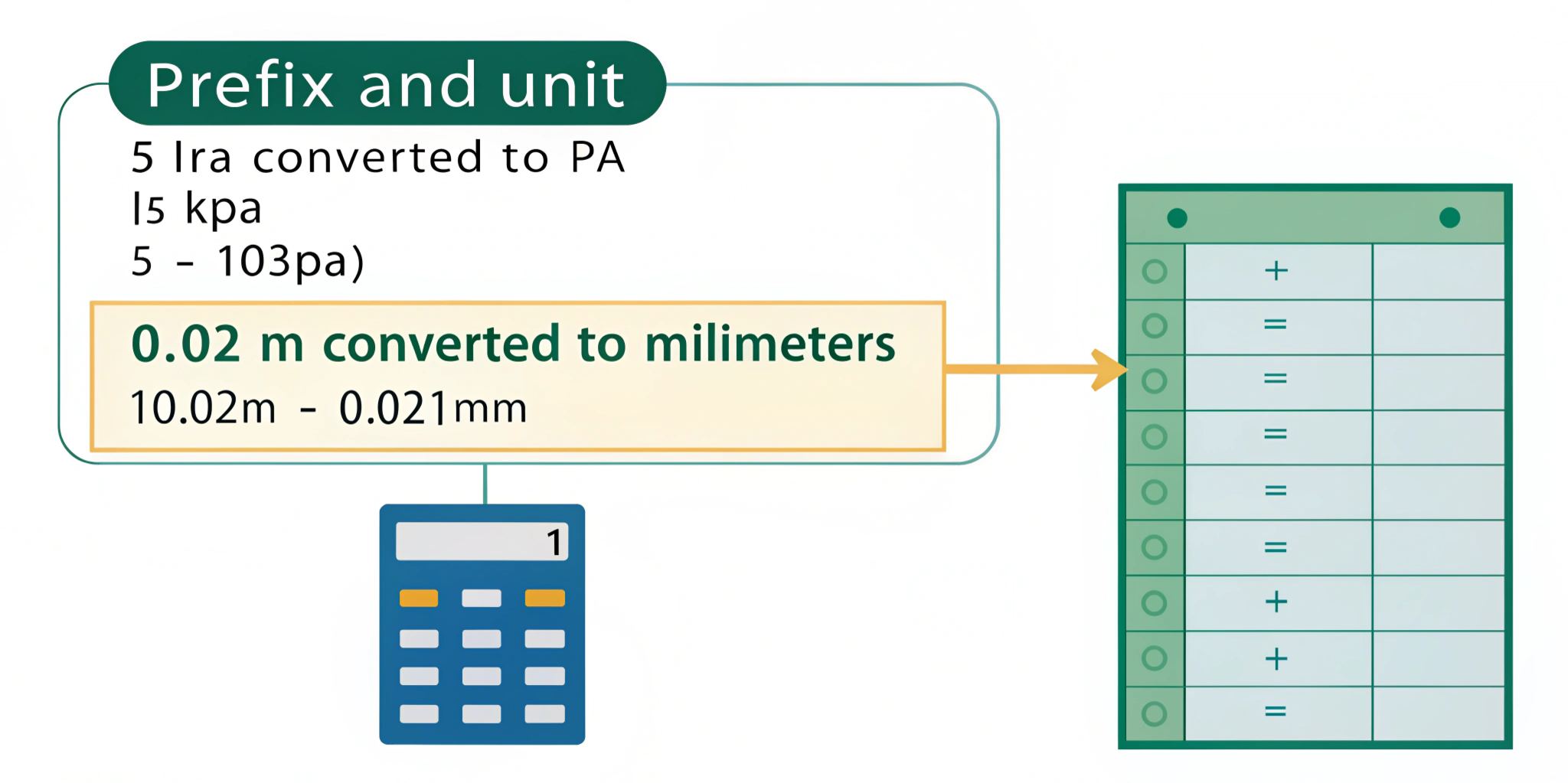
Conversion within SI is straightforward. First identify the prefix and unit. Next, shift the decimal point by the prefix factor. For example, to convert 5 kPa to Pa:
5 kPa = 5 × 10³ Pa = 5000 Pa
Similarly, to express 0.02 m as milimeters:
0.02 m = 0.02 × 10³ mm = 20 mm
Because all factors are powers of ten, you avoid complex multipliers.