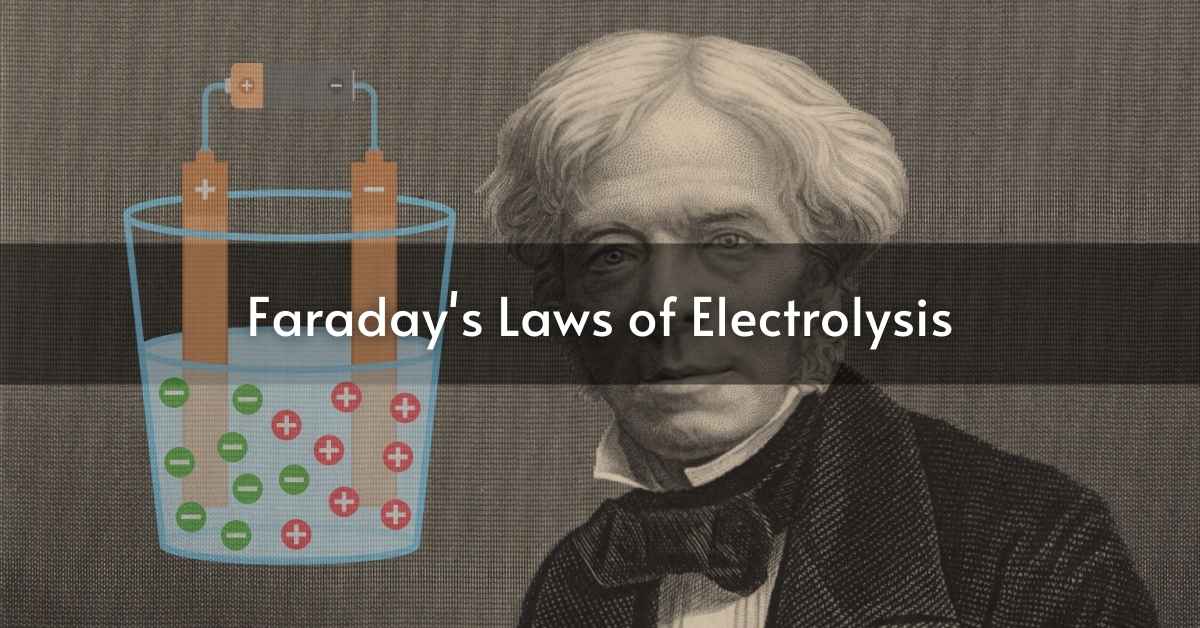
Electrolysis is an application of electrical energy to provoke a chemical reaction that does not spontaneously occur. This is an essential process in several fields such as electroplating and metal extraction. Thus, the laws of electrolysis are important for advanced students in the field of chemistry. As a result, a leading scientist named Michael Faraday introduced two major laws of electrolysis. These are called Faraday’s First and Second Laws of Electrolysis, which explain how chemical energy derives from electrical energy.
Faraday’s First Law of Electrolysis
According to Faraday’s First Law, the quantity of substance deposited or of gas liberated at an electrode during electrolysis is directly proportional to the quantity of electric charge passed through the electrolyte. This relationship can be symbolically represented as:-
M ∝ Q
Where:
- M = mass of the substance deposited or liberated
- Q = total electric charge passed through the electrolyte
This simply indicates that the more quantity of electric charge passed through the solution, the greater the mass of substance deposited will be.
Understanding Charge and Current
The relationship between charge (Q), current (I), and time (t) is given by the formula:
Q = I × t
Where:
- I = current in amperes
- t = time in seconds
Hence, the total charge that has passed through the electrolyte is simply the product of the current and the time for which it flows.
Electrochemical Equivalent
For the measurement of the mass deposited, Faraday introduced the term electrochemical equivalent (Z), which is defined by
Z = E/F
Where,
- E = equivalent weight of the substance
- F = Faraday’s constant ≈ 96500 coulombs per mole of electrons
Therefore, we can represent the mass (M) deposited as:
M = Z × Q
Using the charge equation, we get
M = Z × I × t
This equation will enable one to find the mass of substance deposited during an electrolysis process, in case the current and time as well as the electrochemical equivalent are known.
Faraday’s Second Law of Electrolysis
Faraday’s Second Law is a continuation of the first. It describes the masses deposited during electrolysis when the same quantity of electricity is passed through various electrolytes. It states:
The mass of a substance deposited is directly proportional to its equivalent weight when the same amount of electric charge is passed through different electrolytes.
This can be represented as a formula:
M₁/M₂ = E₁/E₂
Where:
- M₁ and M₂ are the mass of the depositing substance
- E₁ and E₂ are the equivalent weight of the concerned substance
This law predicts the result of electrolysis in different solutions so that chemists can easily calculate the amount of each substance deposited using its equivalent weights.
Applications of Faraday’s Laws
Faraday’s laws of electrolysis aren’t merely theoretical but useful also in many aspects. For example:
- Electroplating: This entails coating an object using a metal layer thereby giving it a better and much more resistant outlook to rusting.
- Metal Extraction: It helps extract metals from their ores, such as extraction of aluminium from its ore bauxite.
- Batteries: There, knowledge about the working of electrolysis is basic because chemical energy is converted into electrical energy.
Conclusion
Faraday’s laws of electrolysis explain the relationship between electricity and chemical reactions in simple terms. These are important both in the educational sectors and in industries. If the students and professionals learn these ideas the proper way, then they can much better understand how such electrochemical processes work and how they influence our world.
FAQs
What is Faraday’s law of electrolysis?
Faraday’s law of electrolysis: The amount obtained in the electrolysis process is directly proportional to the amount of electric charge passed through the electrolyte.
What is Faraday’s first law of electrolysis?
Faraday’s first law of electrolysis states that the mass of substance deposited or dissolved is proportional to the total electric charge passed through electrolyte.
How do you describe Faraday’s law?
The Faraday law says that the electric charge is the quantity that causes more material to deposit or dissolve in the process of electrolysis.
How can Faraday’s law of electrolysis be verified?
You can verify Faraday’s law through an experiment of electrolysis and determining the mass of material deposited through different electric charges.
What is Faraday’s second law?
Faraday’s second law: Mass of several substances deposited from the same quantity of electricity is directly proportional to the chemical equivalent weights of the different substances.
What is the formula of Faraday’s electrolysis?
The formula is: m = E x I x t /96,485
Where m is the mass of substance, Q is the charge, M is the molar mass, F is Faraday’s constant, and z is the number of electrons.
Read More – Millman’s Theorem: Simplifying Complex Circuit Analysis